EXOGEN and Fracture Healing in Uncertain Times
Dr Zura, Orthopaedic Surgeon LSU – LSU Health Sciences Center New Orleans
EXOGEN Accelerates Healing of Fractures in Smokers
View the About EXOGEN video for an overview of the EXOGEN device.
Casting instructions for all installations
EXOGEN Patient Brochure
Introduction to the EXOGEN Ultrasound Bone Healing System with, answers to frequently asked questions.
EXOGEN Quick Instruction Guide
The guide provides step-by-step instructions to quickly start using your EXOGEN device.
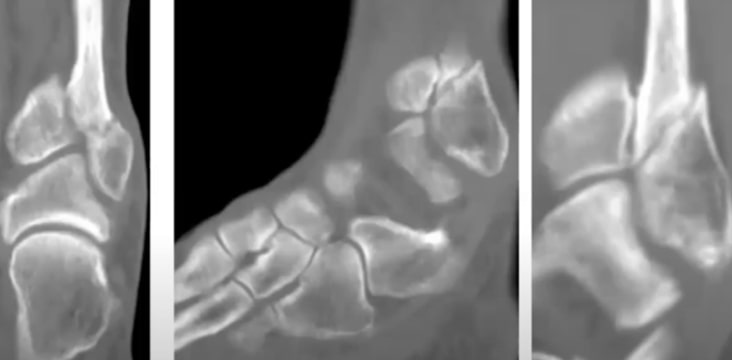
Acute Fractures
- Acceleration of tibia and distal radius fracture healing in patients who smoke.
- Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound.
- Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound. A multicenter, prospective, randomized, double-blind, placebo-controlled study.
Nonunion Fractures
- Indications and results for for the Exogen™ ultrasound system in the management of nonunion: a 59-case pilot study.
- Low-intensity pulsed ultrasound in the treatment of nonunions.
- Low-intensity pulsed ultrasound: effects on nonunions.
- Improved healing response in delayed unions of the tibia with low-intensity pulsed ultrasound: results of a randomized sham-controlled trial.
- The evaluation of the healing rate of subtalar arthrodeses, part 2: the effect of low-intensity ultrasound stimulation.
- Compound high-energy limb fractures with delayed union: our experience with adjuvant ultrasound stimulation (exogen).
- Failure of surgery for scaphoid non-union is associated with smoking.
- Treatment of chronic (>1 year) fracture nonunion: heal rate in a cohort of 767 patients treated with low-intensity pulsed ultrasound (LIPUS).
Other
- Mode & mechanism of low intensity pulsed ultrasound (LIPUS) in fracture repair.
- Prolonged endochondral bone healing in senescence is shortened by low-intensity pulsed ultrasound in a manner dependent on COX-2.
- Sources of information influencing decision-making in orthopaedic surgery – an international online survey of 1147 orthopaedic surgeons.
- Design evolution enhances patient compliance for low-intensity pulsed ultrasound device usage.
The EXOGEN Performance Program is a program that refunds patients their out-of-pocket payment for EXOGEN if progression of healing is not shown per criteria below. It is also designed to help reinforce patients’ adherence to the prescribed treatment.
Criteria
All patients who have purchased an EXOGEN designed for the treatment of a fracture, which was prescribed by a qualified physician to treat a stable, non-displaced, established nonunion,* delayed union or acute fracture with a fracture gap less than 10 millimeters (excluding vertebra and skull fractures) are eligible. All patients will automatically be enrolled in the program.
Patients must treat their fracture with EXOGEN per product instructions, for a minimum of 120 consecutive days and achieve a minimum adherence of at least 90%.
Evaluation
To be eligible, your prescribing physician must determine that your bone has not healed, or progressed to a bony union. The prescribing physician must provide two X-rays of your affected bone, one prior to using EXOGEN, and another taken after 120 days or more of EXOGEN treatment that shows a lack of bony union.
The EXOGEN device contains an internal patient usage monitor that records the date, time and duration of each treatment session. This monitor will be used by Bioventus to confirm that at least 90% treatment adherence is met.
Exclusions
- Fracture types:
- Unstable
- Displaced
- Greater than 10-millimeter fracture gap
- Vertebra and skull
- Pathological
- Modified or altered devices
- Patient treated with EXOGEN 4000+ model
- The EXOGEN Performance Program is void if alternative interventions occur during the 120-day treatment period. If alternative intervention is needed, a new 120-day treatment period begins
- EXOGEN must be purchased and received by the patient directly from an authorized distributor of EXOGEN.
- The EXOGEN Performance Program applies only to patients for whom the device was prescribed
- Any other costs associated with the purchase – only the patient’s out-of-pocket payment to the authorized distributor will be refunded
If you want to verify your eligibility for the program, please visit the contact us page for the distributor’s information. Bioventus does not handle these claims directly. You will need to contact the distributor exclusively.
Claims must be received within one year of first EXOGEN treatment date.
Bioventus or the authorized distributor reserves the right to amend or cancel the program at any time.
- The EXOGEN Post-Market Data App contains real-world bone healing statistics, which can be used to benchmark your specific patients.
- It’s our free smartphone app that automatically tells you the heal rate associated with common fractures, factoring in patient risk factors and other relevant clinical considerations.
- Let the EXOGEN Post-Market Data App help you find the next appropriate patient.
Summary of Indications for Use
The EXOGEN Ultrasound Bone Healing System is indicated for the non-invasive treatment of established nonunions* excluding skull and vertebra. The EXOGEN device has also been reported as effective as an adjunctive non-invasive treatment of established nonunions in patients:
- With internal or external fracture fixation hardware present. EXOGEN cannot penetrate metal and therefore should not be applied directly over hardware.
- Undergoing treatment for infection at the fracture site. EXOGEN is not intended to treat the infection.
- Believed to have diminished bone quality. EXOGEN is not intended to treat diminished bone quality.
In addition, EXOGEN is indicated for accelerating the time to a healed fracture for fresh, closed, posteriorly displaced distal radius fractures and fresh, closed or Grade I open tibial diaphysis fractures in skeletally mature individuals when these fractures are orthopedically managed by closed reduction and cast immobilization.
There are no known contraindications for the EXOGEN device. Safety and effectiveness have not been established for individuals lacking skeletal maturity, pregnant or nursing women, patients with cardiac pacemakers, on fractures due to bone cancer, or on patients with poor blood circulation or clotting problems. Some patients may be sensitive to the ultrasound gel.
*A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
Full prescribing information can be found in product labelling, at EXOGEN.com, or by calling Customer Service at 1-800-836-4080.