How it works
The Science of Bone Healing with EXOGEN
You can’t hear it working, but EXOGEN’s effects on bone healing have been evaluated in multiple clinical studies.*1-3,5,13-31
EXOGEN Mechanism of Action
The EXOGEN Bone Healing System uses safe, painless, low-intensity pulsed ultrasound (LIPUS) to amplify natural bone healing three ways:
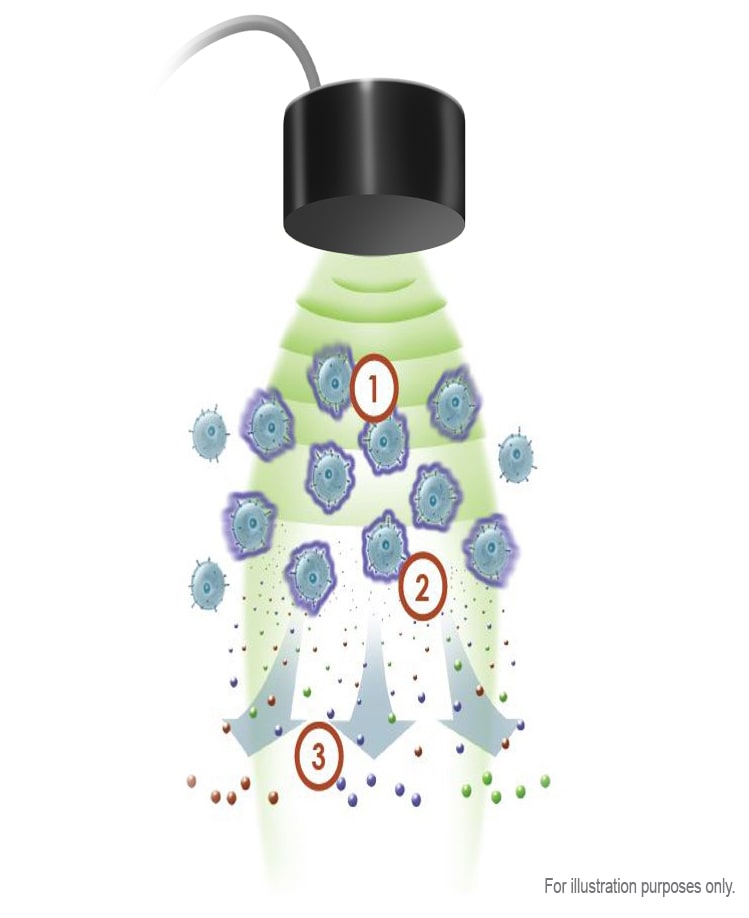
EXOGEN Enhances Bone Healing at Every Stage†35
Within minutes of a bone fracture, the body begins a process to repair the damage. Healing a fracture involves several distinct stages. EXOGEN jumpstarts bone healing by stimulating your body’s natural healing process.
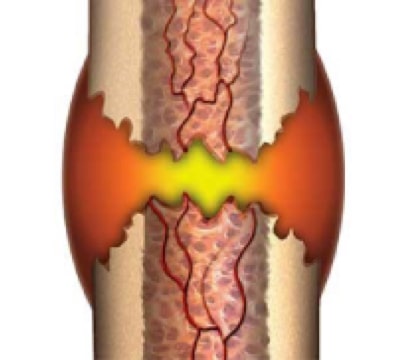
1. Inflammation
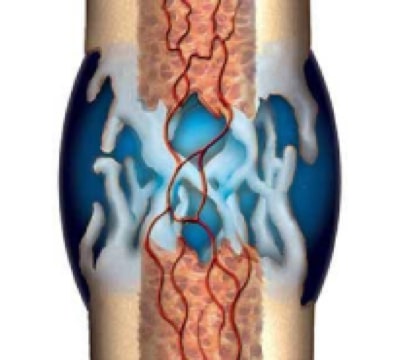
3. Hard Callus
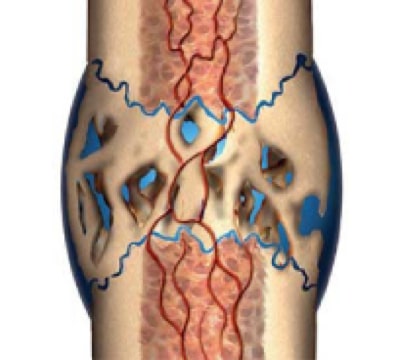
2. Soft Callus
4. Bone Remodeling
†In vivo data is not representative of clinical results
* As of 6/17/21, these studies, which reflect the body of evidence of the bone stimulator EXOGEN, include evaluations of applications outside the approved label. Assignment of evidence levels was based on the updated level of evidence rating system in the Oxford Level of Evidence Criteria.
†In vivo data is not representative of clinical results
Summary of Indications for Use
The EXOGEN Ultrasound Bone Healing System is indicated for the non-invasive treatment of established nonunions* excluding skull and vertebra. The EXOGEN device has also been reported as effective as an adjunctive non-invasive treatment of established nonunions in patients:
- With internal or external fracture fixation hardware present. EXOGEN cannot penetrate metal and therefore should not be applied directly over hardware.
- Undergoing treatment for infection at the fracture site. EXOGEN is not intended to treat the infection.
- Believed to have diminished bone quality. EXOGEN is not intended to treat diminished bone quality.
In addition, EXOGEN is indicated for accelerating the time to a healed fracture for fresh, closed, posteriorly displaced distal radius fractures and fresh, closed or Grade I open tibial diaphysis fractures in skeletally mature individuals when these fractures are orthopedically managed by closed reduction and cast immobilization.
There are no known contraindications for the EXOGEN device. Safety and effectiveness have not been established for individuals lacking skeletal maturity, pregnant or nursing women, patients with cardiac pacemakers, on fractures due to bone cancer, or on patients with poor blood circulation or clotting problems. Some patients may be sensitive to the ultrasound gel.
*A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
Full prescribing information can be found in product labelling, at EXOGEN.com, or by calling Customer Service at 1-800-836-4080.
* As of 6/17/21, these studies, which reflect the body of evidence of the bone stimulator EXOGEN, include evaluations of applications outside the approved label. Assignment of evidence levels was based on the updated level of evidence rating system in the Oxford Level of Evidence Criteria.
†In vivo data is not representative of clinical results


