The Science Behind EXOGEN
The EXOGEN Bone Healing System uses safe, painless, low-intensity ultrasound waves to amplify your patient’s natural bone repair processes.
Mechanism of Action
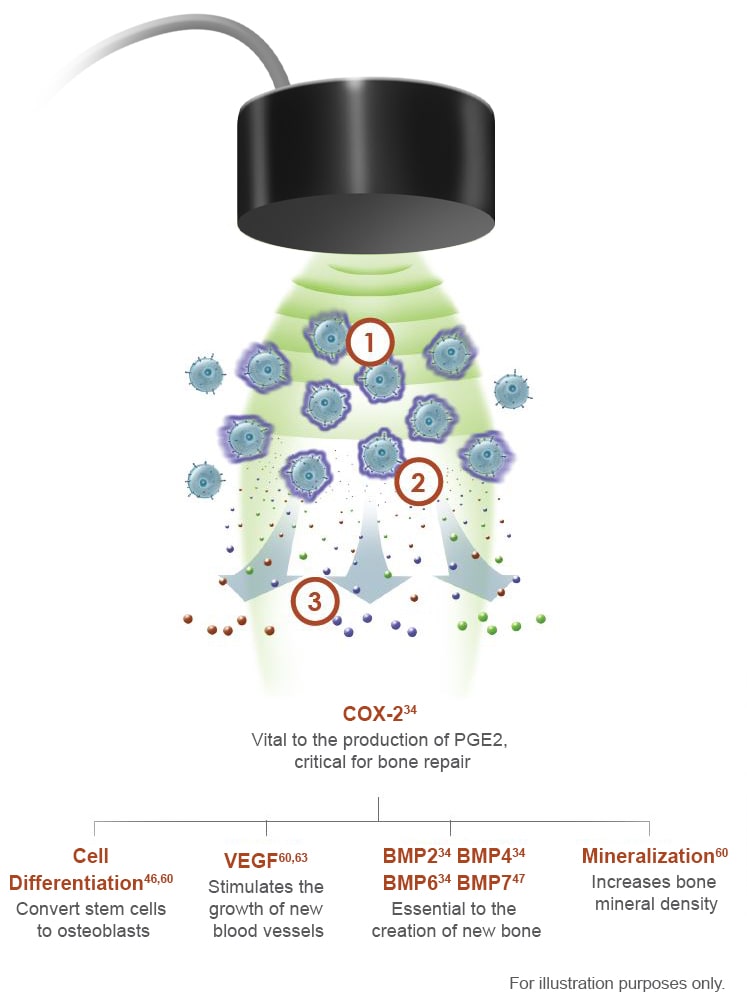
EXOGEN has multiple Level 1 and 2 clinical studies*1-3,5,13-31
*These studies, which reflect the body of evidence of the bone stimulator EXOGEN, include evaluations of applications outside the approved label. Assignment of evidence levels was based on the updated level of evidence rating system in the Oxford Level of Evidence Criteria.
Indications for Use
EXOGEN is indicated for the non-invasive treatment of osseous defects (excluding vertebra and skull) that includes the treatment of delayed unions, nonunions,† stress fractures and joint fusion. EXOGEN is also indicated for the acceleration of fresh fracture heal time, repair following osteotomy, repair in bone transport procedures and repair in distraction osteogenesis procedures.
There are no known contraindications for the EXOGEN device. Safety and effectiveness have not been established for individuals lacking skeletal maturity, pregnant or nursing women, patients with cardiac pacemakers, on fractures due to bone cancer, or on patients with poor blood circulation or clothing problems. Some patients may be sensitive to the ultrasound gel.
Full prescribing information can be found in product labeling, at https://www.exogen.com/, or by calling Bioventus Customer Service.
†A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
*The clinical relevance of in vivo findings is unknown
Summary of Indications for Use
EXOGEN is indicated for the non-invasive treatment of osseous defects (excluding vertebra and skull) that includes the treatment of delayed unions, nonunions,* stress fractures and joint fusion. EXOGEN is also indicated for the acceleration of fresh fracture heal time, repair following osteotomy, repair in bone transport procedures and repair in distraction osteogenesis procedures.
There are no known contraindications for the EXOGEN device. Safety and effectiveness have not been established for individuals lacking skeletal maturity, pregnant or nursing women, patients with cardiac pacemakers, on fractures due to bone cancer, or on patients with poor blood circulation or clotting problems. Some patients may be sensitive to the ultrasound gel.
Full prescribing information can be found in product labeling, at EXOGEN.com, or by calling Bioventus Customer Service.
*A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
