Military personnel and veterans deserve the best!
Give their fractures a healing advantage—from day one of EXOGEN’s 20-minute treatments.
For nonunions and indicated* fresh fractures: Prescribe EXOGEN
EXOGEN uses safe, painless, effective, low-intensity pulsed ultrasound to stimulate the body’s natural healing process.39,68
Choose the FDA-approved bone healing device with 30 years of proven bone healing:71
- 86% heal rate for nonunion fractures3,35,70
- Up to 58 days faster healing of indicated* fresh fractures1
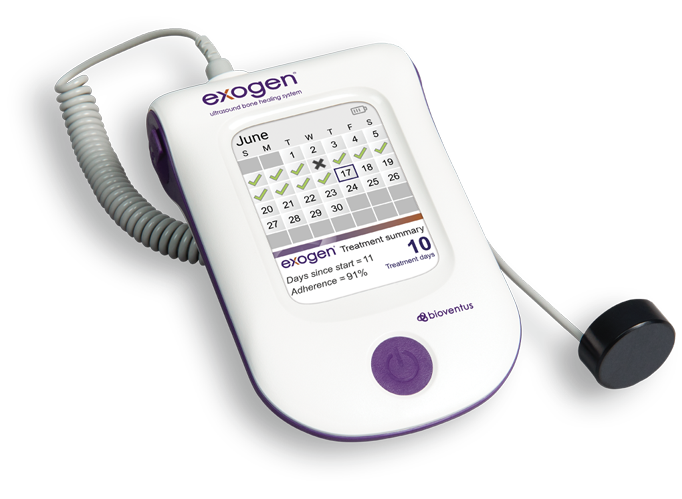
- 91% clinically proven treatment adherence rate5
- The #1 bone healing device prescribed for fracture stimulation (for long bones in the United States)11
By promoting fracture healing, EXOGEN can also help patients avoid further surgery and return to their normal activities faster.1,29,35,52
EXOGEN treatment even helps improve fracture healing in patients with common risk factors, such as smoking or advanced age.9,17
Easy to use and requiring only 20 minutes per day, EXOGEN has a treatment-tracking calendar that makes adherence convenient for patients and verifiable for clinicians.68,69
Ready to prescribe EXOGEN?
Or just have questions?
We’re here to help!
*Summary of Indications for Use
†The clinical relevance of in vivo findings is unknown.
Summary of Indications for Use:
The EXOGEN Ultrasound Bone Healing System is indicated for the non-invasive treatment of established nonunions‡ excluding skull and vertebra. The EXOGEN device has also been reported as effective as an adjunctive non-invasive treatment of established nonunions in patients:
- With internal or external fracture fixation hardware present. EXOGEN cannot penetrate metal and therefore should not be applied directly over hardware.
- Undergoing treatment for infection at the fracture site. EXOGEN is not intended to treat the infection.
- Believed to have diminished bone quality. EXOGEN is not intended to treat diminished bone quality.
In addition, EXOGEN is indicated for accelerating the time to a healed fracture for fresh, closed, posteriorly displaced distal radius fractures and fresh, closed or Grade I open tibial diaphysis fractures in skeletally mature individuals when these fractures are orthopedically managed by closed reduction and cast immobilization.
There are no known contraindications for the EXOGEN device. Safety and effectiveness have not been established for individuals lacking skeletal maturity, pregnant or nursing women, patients with cardiac pacemakers, on fractures due to bone cancer, or on patients with poor blood circulation or clotting problems. Some patients may be sensitive to the ultrasound gel.
‡A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
Full prescribing information can be found in product labeling, at EXOGEN.com, or by calling Customer Service at 1-800-836-4080.
All revisions of the Instructions for Use can be made available upon request. Contact [email protected] or call 1-800-836-4080 to request an electronic copy.